[Synobio Technology] Plasmid DNA Preparation

For years, Synbio Technologies has provided comprehensive plasmid DNA preparation services to our customers from research institutes to both biotechnology and biopharmaceutical industries. We have developed and perfected a manufacturing pipeline designed to meet the requirements of the Good Manufacturing Protocol (GMP). These requirements guarantee both in-process controls and final product quality control, to ensure that the highest quality product will be delivered to our customers.
All plasmids we manufactured are free of animal-derived materials and can contain low levels of endotoxin (<100EU/mg, on request). This process is designed to meet our customers’ diverse downstream applications such as transfection, antibody preparation, vaccine, and gene-therapy research, etc.
Competitive Advantages
- Express and Guaranteed Plasmid Preparation Services: USA based manufacturing allows high quality products and fast delivery.
- Highly Customized: Microgram-to-gram-scale quantity can meet various customers’ needs.
- Low Endotoxin Level: Animal-derived materials free, <100EU/mg, on request.
- Strict Quality Control: ISO9001 & ISO13485 quality management, complete and detailed manufacturing documentation.
- Intellectual Property (IP) Protection: All our customers’ intellectual project related rights are fully respected and protected.
Experimental Procedure
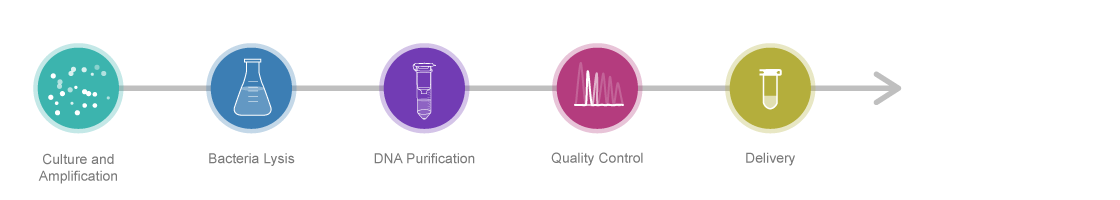
Standard PlasmidPreparation PackageResearch GradeTransfection Grade
| Endotoxin Level | NA | <100EU/mg* | |
| Quantity | 0.1 mg to gram level | 1 mg to gram level | |
| Turnaround time | Starting at |
Starting at |
|
| Quality Control Methods | Restriction enzyme analysis |
|
|
| Recommended Applications |
|
|
|
| Standard Delivery Package Contains | Prepared plasmid DNA, Certificate of analysis (COA) and QC report | ||
*Optional endotoxin level: < 100EU/mg, <30EU/mg, <5EU/mg.
'Synbio Tech' 카테고리의 다른 글
| [Synbiotech] Immune Checkpoint Proteins (0) | 2022.05.09 |
|---|---|
| [Synbio Technolgies] HTP Antibody Gene Synthesis (0) | 2021.06.03 |
| [Synbio Technologies] Standard DNA Oligos (0) | 2021.02.04 |


